Benefits of Stem Cell Therapy for Multiple Sclerosis
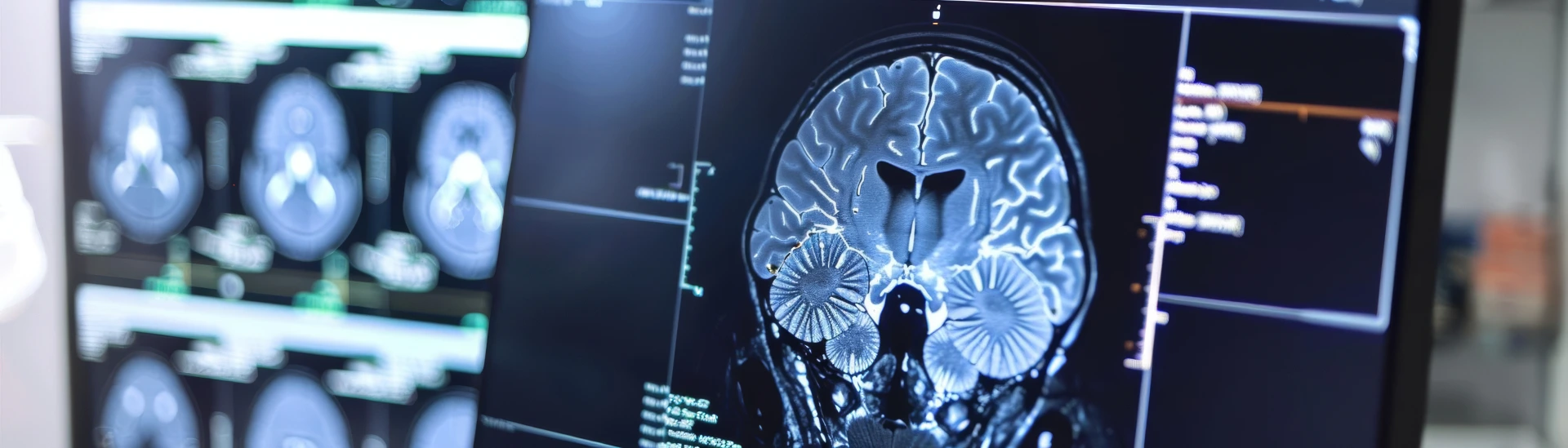
- Neuroregeneration: Stem cell therapy helps regenerate damaged nerve cells, promoting the repair of myelin and restoring normal nerve function in MS patients.
- Reduction in Inflammation: Stem cells have powerful anti-inflammatory effects, which help reduce the chronic inflammation associated with Multiple Sclerosis.
- Improved Mobility: Stem cell therapy can improve muscle strength, coordination, and overall mobility, helping patients regain independence.
- Decreased Muscle Spasticity: Stem cells can reduce muscle stiffness and spasticity in MS patients by addressing nerve damage and improving neural function.
- Improved Cognitive Function: Stem cell therapy may positively impact memory, concentration, and other cognitive functions often affected by MS.
- Enhanced Quality of Life: Stem cell therapy can improve the overall quality of life for MS patients by relieving symptoms and improving functionality.
- Potential for Disease Modulation: Stem cells have the potential to slow or even halt the progression of MS by promoting tissue repair and immune system balance.
- Reduced Dependency on Medications: Some patients may find a reduction in their need for conventional MS medications, with stem cell therapy offering a natural alternative.
- Long-Term Benefits: Stem cell therapy offers lasting results by promoting continuous repair and regeneration of nerve tissues, leading to sustained symptom relief over time.
The Most Advance Treatments Available
Stem cell therapy has helped many by promoting healing, reducing inflammation, and regenerating tissue. Patients with autoimmune diseases, injuries, and neurodevelopmental disorders report improved mobility, less pain, and better cognitive function. While research continues, it offers hope for innovative treatments.
Citations & Scientific References
View our medical studies and published studies that support the science behind stem cell therapy.
Clinical Studies- IV/IT hUC-MSCs Infusion in RRMS and NMO: A 10-Year Follow-Up Study. Zhengjuan Lu, Lin Zhu, Zhuo Liu, Jiayong Wu, Yun Xu, Cun-Jin Zhang – https://www.frontiersin.org/journals/neurology/articles/10.3389/fneur.2020.00967/full
- MEsenchymal StEm cells for Multiple Sclerosis (MESEMS): a randomized, double blind, cross-over phase I/II clinical trial with autologous mesenchymal stem cells for the therapy of multiple sclerosis. Antonio Uccelli, Alice Laroni, Lou Brundin, Michel Clanet, Oscar Fernandez, Seyed Massood Nabavi, Paolo A. Muraro, Roberto S. Oliveri, Ernst W. Radue, Johann Sellner, Per Soelberg Sorensen, Maria Pia Sormani, Jens Thomas Wuerfel, Mario A. Battaglia & Mark S. Freedman on behalf of the MESEMS study group – https://trialsjournal.biomedcentral.com/articles/10.1186/s13063-019-3346-z
- Clinical feasibility of umbilical cord tissue-derived mesenchymal stem cells in the treatment of multiple sclerosis. Neil H. Riordan, Isabela Morales, Giselle Fernández, Nicole Allen, Neal E. Fearnot, Michael E. Leckrone, Dedra Jones Markovich, Darla Mansfield, Dorita Avila, Amit N. Patel, Santosh Kesari & Jorge Paz Rodriguez – https://translational-medicine.biomedcentral.com/articles/10.1186/s12967-018-1433-7
- Phase I Trial of Intrathecal Mesenchymal Stem Cell-derived Neural Progenitors in Progressive Multiple Sclerosis. Violaine K Harris, James Stark, Tamara Vyshkina, Leslie Blackshear, Gloria Joo, Valentina Stefanova, Gabriel Sara, Saud A Sadiq – https://pubmed.ncbi.nlm.nih.gov/29449193/
Other Neurological Disorders Therapies
Hear What Our Clients have to say!
Averaging 5 Stars across multiple platforms — TOP RATED
The Process
Day 1: US ➜ Mexico
- Receive the procedure at the clinic.
- Go back to the hotel and rest (you can stay in a Tijuana hotel or hotel in San Diego).
Day 2: Travel back home
- Check out of your hotel.
- Meet your driver outside the hotel for a smooth ride to San Diego International Airport.
- Arrive at the airport in time to catch your flight back home.
Multiple Sclerosis FAQ's
You have questions, we have answers.
While stem cell therapy is not a cure, it can significantly improve symptoms, reduce inflammation, and enhance mobility and cognitive function, offering patients a better quality of life and potentially slowing the disease’s progression.
Patients typically begin to see improvements within a few months, with motor skills, coordination, and reduced spasticity among the first areas to improve. Long-term benefits continue to evolve.
Stem cell therapy is considered safe, but, like any medical procedure, it carries some risks, such as temporary swelling or discomfort at the injection site. However, serious side effects are rare when performed by qualified professionals.
The number of treatments varies depending on individual needs and responses. Many patients experience significant benefits from a single treatment, though some may require follow-up treatments to maintain or enhance results.